Invited Talks
|
Transcatheter Aortic Valve Thrombosis: Mechanism, Prediction, and Prevention
|
Ali N. Azadani
The DU Cardiovascular Biomechanics Lab
University of Denver, CO, USA
|
Transcatheter aortic valve replacement (TAVR) is a rapidly expanding alternative to surgical aortic valve replacement (SAVR) in patients with severe symptomatic aortic stenosis. Subclinical leaflet thrombosis associated with reduced leaflet motion is an increasingly recognized complication following TAVR. It has been recently demonstrated that leaflet thrombosis is significantly more common in transcatheter aortic valves (TAVs) than surgical bioprosthetic aortic valves. In addition, leaflet thrombosis has been observed following valve-in-valve (ViV) procedures. Patients with transcatheter aortic valve (TAV) leaflet thrombosis have a higher incidence of strokes and transient ischemic attacks. Therapeutic anticoagulation with warfarin, as compared with dual antiplatelet therapy, was associated with a decreased incidence of reduced leaflet motion in the patients. However, bleeding is a serious complication of the use of the anticoagulation therapy. Therefore, it is of the utmost importance to investigate the predisposing factors affecting TAV leaflet thrombosis from a fundamental perspective. We have implemented an integrative approach using experimental and computational techniques to determine the underlying mechanism of leaflet thrombosis in TAVs and identify the predisposing factors that contribute to the development of leaflet thrombosis. Our main hypothesis was that geometric confinement of TAVs, introduced by the calcified native valves in TAVR or by the degenerated bioprosthesis in ViV, disturbs the natural flow field between the leaflets and aortic sinuses, increases the blood residence time on the TAV leaflets, and consequently increases the likelihood of thrombogenesis on the leaflets. Regions of blood stasis promote elevated transport of blood components to the biomaterial surface and provide an opportunity for platelets and blood proteins to accumulate to critical concentrations, leading to thrombosis.
|
|
Controlling protein-surface interactions in blood-material contact: towards a fibrinolytic surface
|
John Brash1, Hong Chen2, Dan Li2
1. McMaster University, Hamilton, Ontario, Canada
2. Soochow University, Suzhou, China
|
This presentation will touch briefly on several topics related to blood compatibility, blood-material interactions and the design of thromboresistant surfaces. These include plasma protein adsorption, platelet adhesion, protein resistant surfaces, anticoagulant surfaces, and fibrinolytic surfaces. The behaviour of proteins at interfaces is an important underlying theme of our work, and for a number of years we have followed a two-point principle for the design of blood compatible surfaces based on controlling protein-surface interactions: (1) preventing nonspecific protein interactions (“the enemy”), and (2) promoting the specific interactions of a target protein (or proteins) expected to provide appropriate bioactivity, e.g. anti-bacterial, anti-inflammatory, anti-thrombotic. The focus in this presentation will be on surfaces having clot-lysing properties based on their ability to “capture” endogenous plasminogen and tissue plasminogen activator (t-PA), the key players in the fibrinolytic system. This represents a relatively unexplored approach to the serious, and seemingly intractable, problem of foreign surface-induced thrombosis, and is in contradistinction to the more common “prevention” approach which has so far failed to provide a solution.
|
|
Integrating the platelet signaling network and the hemostatic response to injury.
|
Lawrence F. Brass1, Peisong Ma1, Maurizio Tomaiuolo1, John Welsh1, Scott Diamond2 and Timothy Stalker1
1. Department of Medicine, University of Pennsylvania, Philadelphia, PA, USA
2. Department of Chemical Engineering, University of Pennsylvania, Philadelphia, PA, USA
|
When viewed in real time in the microcirculation, the hemostatic response to penetrating injuries produces a thrombus comprised mainly of activated platelets with relatively little fibrin. The architecture of the thrombus that is formed is heterogeneous, with a core of fully activated, densely-packed platelets overlaid by a shell of less activated, loosely-packed platelets. In this setting, the normally free movement of soluble molecules in the gaps between platelets slows to a crawl, and thrombin activity and fibrin deposition become largely confined to the thrombus core. At a larger scale, a similar thrombus forms in the femoral artery and vein after injury, although with key differences due in part to the greater thickness of the vessel wall. Here we will consider how thrombus structure reflects the distribution of agonist gradients within the thrombus, exposing platelets in different regions of the thrombus to different combinations of agonists. The role of the platelet signaling network is to integrate these varied inputs within individual platelets, producing a measured response from the thrombus as a whole. Accomplishing this calls for close regulation of both signal strength and signal duration. How this is accomplished at the molecular level in different regions of the thrombus will be the subject of this presentation.
|
|
|
|
Thrombogenicity testing of polymers: round-robin study to assess inter-center variability
|
Steffen Braune1, Cladia Sperling,2 Manfred F. Maitz,2 Ulrich Steinseifer,3 Johanna Clauser,3 Bernhard Hiebl,4 Stefanie Krajewski,5 Hans-Peter Wendel,5 Friedrich Jung1
1. Helmholtz-Zentrum Geesthacht und Berlin-Brandenburger Centrum für Regenerative Therapien, Teltow, Germany
2. Max Bergmann Center of Biomaterials Dresden, Leibniz Institute of Polymer Research Dresden, Dresden, Germany
3.Department of Cardiovascular Engineering, Institute of Applied Medical Engineering Helmholtz-Institute, RWTH Aachen University, Aachen, Germany
4.Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Foundation, Hannover, Germany
5.Department of Thoracic and Cardiovascular Surgery, University Medical Center Tübingen, Tübingen, Germany
|
Clinical use of cardiovascular devices is continuously increasing along with concerns on the thrombogenicity or hemocompatibility of these implants. The lack of standardization in the in vitro testing of new implant materials has led to a situation where definite specifications about test panels and protocols are lacking and inter-study comparisons are rather impossible.
Here, we report about a prospective, randomized and double-blind multicenter trial demonstrating that standardization of in vitro test protocols allows a reproducible assessment of platelet adhesion and activation from platelet rich plasma as indicators of the thrombogenicity of cardiovascular implant materials.The stringent standardization of a static platelet adhesion test resulted in a laboratory independent scoring of the polymers; the materials were evaluated in all laboratories in the same order for their thrombogenicity. While poly(dimethyl siloxane) showed very reduced platelet adhesion and activation, the density of cells and the degree of activation were highest on poly(tetrafluoro ethylene). Polyethylene terephthalate showed intermediate values.
The results of this study show that inter-laboratory and inter-study comparisons of the thrombogenicity testing of blood-contacting biomaterials can be achieved by a stringent standardization of the test protocol. Future perspectives, how improved standardization in the in vitro testing of new implant materials could be realized, will be discussed in the talk.
|
|
|
|
Coagulation under flow: Role of platelet polyphosphate and contact activation
|
Scott L. Diamond, Shu Zhu, Brad Herbig
Department of Chemical Engineering, University of Pennsylvania, Philadelphia, PA, USA
|
RATIONAL: In biomedical device thrombosis, the generation of thrombin near a surface typically requires activation of the contact pathway. Additionally, intravascular thrombosis may involve components of the contact pathway such as FXIIa or FXIa.
METHOD: Microfluidic devices allow controlled flow of blood over defined zones of procoagulant surfaces. Pretreatment of blood with low or high level of corn trypsin inhibitor (4 or 40 ug/mL, respectively) allows for weak or strong inhibition of FXIIa.
RESULTS: Kaolin activation of blood under flow: We used a patterned kaolin (0 to 0.3 pg/µm2)/type 1 collagen fibril surface for controlled microfluidic clotting assays. At venous wall shear rate (100 s-1), kaolin accelerated onset of fibrin formation by ~100 sec when compared to collagen alone (250 sec vs. 350 sec), with little effect on platelet deposition. Even with kaolin present, arterial wall shear rate (1000 s-1) delayed and suppressed fibrin formation compared to venous wall shear rate. Extrinsic-Contact crosstalk via platelet polyphosphate: Platelets release polyphosphate which may activate FXIIa or enhance thrombin-feedback activation of FXIa. We found that a polyphosphate inhibitor (PPXbd) reduced fibrin generation at low [TF]wall (0.1 molecules per µm2) but not at zero or high [TF]wall, suggesting a role for polyP distinct from FXIIa activation and requiring low extrinsic pathway participation. Antibody studies supported the role of platelet polyphosphate in enhancing thrombin-feedback activation of FXIa. Direction measurements of thrombin flux from clots under flow: The majority (>85%) of generated thrombin was captured by intrathrombus fibrin since thrombin-antithrombin was largely undetectable in the effluent unless GPRP was added to block fibrin polymerization. With GPRP present, the flux of thrombin increased to ~0.5 × 10-12 nmol/µm2s-1 over the first 500 s of perfusion and then further increased by ~2-3-fold over the next 300 s. The increased thrombin flux after 500 s was blocked by anti-FXIa antibody (O1A6), consistent with thrombin-feedback activation of FXI.
CONCLUSION: Overall, microfluidics allows precise studies of coagulation kinetics and allow exploration of platelet biology in the presence of the extrinsic pathway and contact pathway.
|
|
|
|
Tunable Activation of Platelet-Rich Plasma Using Pulsed Electric Fields.
|
A.L. "Larry" Frelinger III
Harvard Medical School; Center for Platelet Research Studies; Division of Hematology/Oncology, Boston Children's Hospital, Boston, MA, USA.
|
Background: Activation of concentrated platelet-rich plasma (PRP) by pulse electric field (PEF) produces gels and releases growth factors which may be clinically useful for wound healing. The practical utility of PEF-activated PRP may depend on the physical strength of the gels produced and the precise profiles of growth factors released.
Aims: To determine platelet gel strength and the profile of released factors following activation of PRP with different PEF conditions.
Methods: Concentrated PRP was prepared from acid-citrate-dextrose anticoagulated normal donor blood (n=5) in the Harvest SmartPreP2 System, then activated by PEF (PEF A or PEF B) in the presence of 20 mM added CaCl2. Clot formation was evaluated by thromboelastography (TEG) and surface exposure of alpha granule (P-selectin) and T-granule (toll-like receptor 9 [TLR9] and protein disulfide isomerase [PDI]) markers were assessed by flow cytometry. Growth factors were measured by ELISA in supernatants recovered after 15 min activation. Platelet morphology was evaluated by transmission electron microscopy (TEM). Controls included PRP treated with buffer only, CaCl2 only, and thrombin with added CaCl2.
Results: Time to initial clot formation was shorter with thrombin (<1 min) than with PEF A and B (4.4 – 8.7 min) but clot strength (elastic modulus, derived from TEG maximum amplitude) was greater with PEF B than with either thrombin or PEF A (p < 0.05). Supernatants of PRP activated with PEF A had higher epidermal growth factor (EGF) levels than supernatants from PEF B or thrombin-activated PRP (p < 0.05). In contrast, levels of platelet factor 4, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) were similar in supernatants after PEF A, PEF B, and thrombin activation. Platelet surface P-selectin was higher following thrombin activation compared to PEF A or B (p < 0.05). Platelet surface TLR9 and PDI were higher after thrombin activation than after PEF A or PEF B activation (p < 0.05). By TEM, platelets in PEF-treated samples had a distinct morphology and retained a subset of granules.
Conclusion: PEF produces platelet gels as strong as those produced by thrombin, and is tunable to produce growth factor profiles enhanced in specific factors important for different stages of wound healing (e.g., PDGF and VEGF for granulation, EGF for epithelialization). Differences in the levels of EGF in supernatants relative to other growth factors suggests EGF distribution within platelets is different from other growth factors. Moreover, the distinct morphology of PEF-treated platelets and the pattern of growth factor release suggests PEF initiates regulated release of platelet contents rather than non-specific mechanical fracturing.
|
|
|
|
Biomedical applications of Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) imaging.
|
Lara J. Gamble
NESAC/BIO, Department of Bioengineering, University of Washington, Seattle, WA, USA.
|
Time-of-flight mass spectrometry (ToF-SIMS) imaging can provide sub-micron resolution images of cells, tissues, and other biomedically relevant samples with chemical and molecular specificity. These chemically specific images could revolutionize our understanding of biological processes and the interaction between cells/tissues and biomaterials. Applications include spatially identifying chemical changes as a function of an applied stress (delivered drug or biomaterials) or as a result of disease as well enable tracking the spatial distribution of metabolites and lipids. Chemistry of tumor microenvironments, lipid metabolomics relationship to cancer, and tissue repair could be visualized on a cellular level. Similarly, ToF-SIMS images of biomaterials (e.g. polymer scaffolds) can give insight to the biological response to biomaterials.
In this presentation, ToF-SIMS imaging analysis of biological samples will be presented. ToF-SIMS 2D images of cancer tumor tissues will be presented to highlight the utility of ToF-SIMS for investigating chemistry of tissue. Additionally, ToF-SIMS imaging of polymer scaffolds will be presented. Challenges with sample preparation for the ToF-SIMS environment and processing of the large amount of data will be discussed (including multivariate analysis of the ToF-SIMS image data). Overlap of ToF-SIMS images with imaging techniques (optical images and second harmonic generation microscopy) of the same samples will be discussed. Such image comparisons allow researchers to visualize a molecular map that correlates with specific biological features or functions.
|
|
|
|
Platelets, complement and leukocytes – the complex interactions contributing to material-induced thrombogenicity.
|
Maud Gorbet
Department of Biomedical Engineering, University of Waterloo, Canada
|
Cardiovascular devices, such as heart valves, stents and vascular grafts, have increased life expectancy and quality of life of millions of people. However, despite years of research, cardiovascular materials are still not truly blood-compatible and require the use of anticoagulant and anti-platelet therapies to prevent thrombotic complications. Other approaches, such as complement inhibition, have also been considered to improve biocompatibility of cardiovascular devices. The design of blood-compatible biomaterials is a complex problem due to the cascades of interlinked events (protein adsorption, platelet and leukocyte activation/adhesion, activation of complement and coagulation) that take place at the biomaterial surface. While in vitro experiments of blood-material interactions may have short comings as in vitro models cannot entirely mimic the physiological environment, parameters can be controlled and these studies also allow to gain a better understanding of the mechanisms involved in material-induced blood activation.
In this presentation, the interactions between the different components of the blood system and biomaterials will be briefly introduced. Results from in vitro whole blood experiments in the presence of complement inhibitors, such as soluble CR1 (sCR1) and nafamostat mesilate (FUT-175), and model stents or biomaterials will be presented. The role of complement on platelet and leukocyte response to shear and biomaterials will be discussed and will consider the interplay between the various components of our system (blood, biomaterial, in vitro model, inhibitor) and its impact on material-induced thrombogenicity.
|
|
|
|
Nitric Oxide Materials—An Approach To Creating More Hemocompatible Medical Device Coatings
|
Hitesh Handa
University of Georgia, Athens, GA, USA
|
Blood/material interaction is critical to the success of implantable medical devices, ranging from simple catheters, stents and grafts, to complex extracorporeal artificial organs which are used in thousands of patients every day. There are two major limiting factors to clinical application of blood contacting materials: 1) platelet activation leading to thrombosis, and 2) infection. Despite a thorough understanding of the mechanisms of blood–surface interactions, and decades of bioengineering research effort, the ideal non-thrombogenic prosthetic surface remains an unsolved problem. One approach to improving the hemocompatibility of blood-contacting devices is to develop materials that release nitric oxide (NO), a known potent inhibitor of platelet adhesion/activation and also an antimicrobial agent. Healthy endothelial cells exhibit a NO flux of 0.5-4x10-10 mol cm-2 min-1, and materials that mimic this NO release are expected to have similar anti-thrombotic properties. The potential of incorporating NO donor molecules such as diazeniumdiolates or S-nitrosothiols (RSNOs) into various polymers, and their hemocompatibility and antibacterial properties in short-term (4 h) and long-term (9 d) animal models will be discussed.
|
|
|
|
The Role of Platelets in Hemocompatibility Assessments of Medical Devices
|
Lisa K. Jennings1,2 and Benjamin J. Curry1
1. CirQuest Labs, LLC, Memphis, TN, USA
2. The University of Tennessee Health Sciences Center, Memphis, TN, USA
|
Blood compatibility testing of biomaterials and medical devices continues to be a challenging issue to address due to the complexity of blood cells, plasma components, and the various interactions of blood products that can occur during material exposure. Blood platelets play a significant role in determining what materials are considered compatible as they have critical roles in both hemostasis and thrombosis; however, most testing strategies do not consider the secondary and tertiary effects of platelet activation on other blood components that, for example, influence host inflammatory response. Testing should routinely be performed in whole, fresh blood under physiologically relevant flow conditions. Anticoagulation choice, blood donor variability, surface area exposure, and shear stress can impact platelet activation during hemocompatibility evaluations. This presentation will discuss the process of platelet activation, most relevant platelet function assays that should be used for material testing, and the most relevant biomarkers to measure (platelet adhesion and aggregation, PAC-1 and CD62P expression, platelet secretion, etc.) to gain a complete understanding of how platelets interact with artificial surfaces or biomaterials. While platelets are an important, independent contributor, other components of blood should not be discounted when considering a material/device as “hemocompatible,” such as leukocyte activation, complement system activation, thrombin generation, and hemolysis.
|
|
|
|
Thrombosis: Prosthetic Materials & Difficulties with Patency
|
Eugene Langan III
University Medical Group, Greenville Health System, Greenville, SC, USA
|
Dealing with the clinical scenarios of revascularization, the three major factors are inflow, outflow and conduit. These basic “plumbing” factors have become more difficult as clinical techniques continuously improve but the conduit issue does not. Autologous conduit remains the gold standard but still has limitations. Prosthetic conduit needs to improve to avoid thrombosis and maintain patency. Problems, improvements and options will be explored.
|
|
|
|
Methods for visualizing thrombus formation on surfaces in static system and in flow
|
Tomas L. Lindahl
Department of Clinical and Experimental Medicine, Clinical Chemistry, Linköping University, Sweden
|
There is a demand for relevant in vitro thrombosis/hemostasis models in basic research and in drug and biomaterial development.
We have constructed a setup for imaging of coagulation and enabling analysis of initiation and propagation of the coagulation process at surfaces in static conditions. The coagulation or enzymatic activity of a coagulation factor is detected in a cuvette by time-lapse image capture of light scattering from the developing fibrin network. By image processing and computer analysis of the captured image data, both early detection of coagulation at the surface and the propagation phase of coagulation may is in the same experiment. Examples of use for our system and a similar commercial system will be shown.
During platelet accumulation on a vessel injury or foreign surface in flowing blood, a contraction and a segregation of platelets depending on their respective place and role during hemostasis, starts within the first minutes. Thrombus formation, contraction and individual platelet movements can be monitored and evaluated through different approaches. However, none of the existing methods can generate information about both single platelet movements and that of the bulk. Such information would be valuable to understand the dynamics of thrombus contraction. We have developed flow chambers systems to mimic the in vivo conditions of the human vessel during thrombotic processes. To obtain more data we label a minor prat of the platelets, record with time-lapse and z-stack fluorescence microscopy and use image analysis to improve and extend quantitative analysis of thrombus formation. Our novel quantification methods include measurements on platelet movement, contractility, thrombus density and determination of platelet depth within the thrombus, where all measured parameters are determined for each individual platelet with time. Examples on the effect of particular inhibitors affecting the thrombus contraction and thereby the overall stability of the thrombi will be shown.
|
|
|
|
In Vitro Thrombogenicity Testing—FDA Regulatory Considerations and Research Efforts
|
Qijin Lu
US Food and Drug Administration, Silver Spring, MA, USA
|
Thrombosis remains a significant clinical concern for the use of many types of blood-contacting medical devices, including those used short-term as well as for long-term implants. Because device-specific properties (e.g. materials and geometric design) play an important role in thrombosis, it is critical to appropriately assess the thrombogenicity of new devices, or existing devices undergoing material/design changes, prior to clinical use. Thrombogenicity is often assessed as a part of a device safety or functionality study in animals, especially when long term thrombogenicity information is needed. However, animal tests are usually resource-demanding and time-consuming, and the data may have limited clinical predictive value due to various confounding factors and the inherent species differences in blood responses. Although a variety of in vitro test methods for assessing device/material thrombogenicity exist in the literature, most of these test methods are not properly validated and their clinical relevance has not been well-established. At the April 2014 FDA thrombogenicity public workshop, experts from FDA, academia, and industry agreed on the value of in vitro tests but also indicated the need for the validated test methods. Even though the publication of the new FDA biocompatibility guidance document (June 2016) [1] has increased the use of in vitro thrombogenicity tests for regulatory submissions, much work still needs to be done to develop standardized test methods that can be used to effectively evaluate device/material thrombogenicity.
In this talk, an overview of FDA considerations on the evaluation of medical device thrombogenicity and the use of in vitro thrombogenicity test methods will be presented. FDA’s perspectives on how to validate in vitro thrombogenicity test methods will be discussed. In addition, a summary of FDA’s research efforts to qualify and standardize a panel of in vitro test methods for characterizing device/material thrombogenicity will be presented. The goal of the research is to develop standardized, cost-effective and time-saving in vitro test tools to facilitate innovation and improve FDA review efficiency, which will help to bring safe medical devices to patients in a timely manner.
Reference
1. US Food and Drug Administration, Guidance for Industry and Food and Drug Administration Staff—Use of International Standard ISO 10993-1, “Biological evaluation of medical devices –Part 1: Evaluation and testing within a risk management process”. Issued on June 16, 2016.
|
|
|
|
Non-Specific Activation of FXII by Synthetic Surfaces
|
Christopher A Siedlecki1,2, Li-chong Xu1, Yuan Yan1, Erwin A Vogler2,3
1. Department of Surgery, The Pennsylvania State University, Hershey PA, USA
2. Department of Biomedical Engineering, The Pennsylvania State University, University Park PA, USA
3. Department of Materials Science and Engineering, The Pennsylvania State University, University Park PA, USA
|
Contact activation of blood coagulation is a protein-mediated event initiated by contact of a biomaterial with the zymogen Factor XII (Hageman Factor). The model for contact activation has long been believed to be initiated by the formation of a contact activation complex including the proteins FXII, prekallikrein and high molecular weight kininogen, all assembling on surfaces bearing negative charge, with the result being the formation of an αFXIIa that is capable of both amplifying and propagating the cascade, leading to formation of thrombin and ultimately to a cross-linked fibrin mesh. Recent results from our lab suggest that contact activation of FXII can occur in the absence of these other proteins, and on nearly all surfaces, with the resulting activated protein product having 1) procoagulant activity (the ability to form a clot), 2) amidolytic activity (the ability to cleave a chromogen specific for FXIIa) and even 3) inhibitory activity (the ability to inhibit further FXIIa activation/activity). Molecular weight analysis of the activated protein products showed that this activity arises in the absence of the specific cleavage events that yield αFXIIa, suggesting that full length FXII can become activated solely by a surface-induced conformational changes that yield a complex milieu of proteins and presenting each of the various activities.
Although each form of activity can be found after activation by the different surfaces tested, clean glass, methyl-terminated and amine-terminated self assembled monolayers appear to bias the activation products towards the different types of activities, with glass yielding the most procoagulant activity and methyl-terminated SAMS yielding the most inhibitory activity. However, if these activation products are subsequently placed into plasma or purified solutions of prekallikrein/high molecular weight kininogen, there is a resulting formation of the traditional αFXIIa products and all materials eventually show similar levels of all types of activity. From these data, as well as prior studies, we propose that the activation of blood coagulation arises from random structural, non-enzymatic perturbations of FXII, some of which result in activity which is the amplified and propagated down the cascade leading to a fibrin mesh.
|
|
|
|
The Fluoropolymer Hypothesis For Blood Compatibility
|
Buddy D. Ratner
Departments of Bioengineering and Chemical Engineering, University of Washington, Seattle, WA, USA
|
The surface chemistry of polymeric biomaterials can be directly related to specific blood interactions. Starting in the late 1970’s, using a quantitative baboon A-V shunt model we demonstrated that highly hydrophobic materials had much reduced platelet consumption compared to hydrophilic materials. When hydrogels were studied with this A-V shunt model, the higher the water content, the higher the platelet consumption (i.e., the more hydrophobic materials performed better in blood). Also, plasma-fluoropolymer-treated small diameter vascular grafts showed excellent patency in a baboon model compared to control Dacron. More recently, we have been studying adsorbed proteins and their relationship to blood compatibility. Revisiting an old hypothesis, that increased albumin on the surface will lead to improved blood compatibility, we have updated that hypothesis to also relate albumin affinity to surface retention of albumin (tight binding). In addition, we consider the amount of fibrinogen adsorbed to surfaces (in competition with albumin). Based upon a hypothesis by Horbett, adsorbed fibrinogen is the most important activator of platelets. Highly hydrophobic fluoropolymer materials that have shown low platelet reactivity and good performance clinically demonstrate high albumin affinity, low fibrinogen adsorption levels and also tight binding of proteins. Over some 43 years, our group consistently comes up with the same conclusion – in high blood flow areas (high wall shear rate), where the platelets are the primary reactive element in blood, hydrophobic materials show less platelet activation and less thrombosis. We will take this hypothesis to newly developed fluoropolymer materials as part of a program to optimize the good blood contact properties of such polymers.
|
|
|
|
Biological evaluation of medical devices: Selection of tests for interactions with blood: The new 2017 ISO10993-4 standard: what’s new, and will it help to better standardize and define hemocompatibility testing?
|
Michael F Wolf,
Medtronic Inc., Minneapolis, MN, USA;
Convenor, ISO10993-4
|
In the development of new blood-contacting medical devices, the approval pathway is relatively clear if the device material(s), manufacturing method(s), design features, and patient clinical application are similar or identical to those of a legally-marketed and clinically-safe device. However, departures from this scenario, such as introducing new materials, applying alternative manufacturing methods, adding novel device design features, and diverging from the original clinical application, can change this picture. Here, the guideline for recommended preclinical safety testing is the ISO10993-4 international standard. The revision of this standard, expected to be released in mid-2017, attempts to make the decision strategy for testing blood-contacting devices clearer and more scientifically sound.
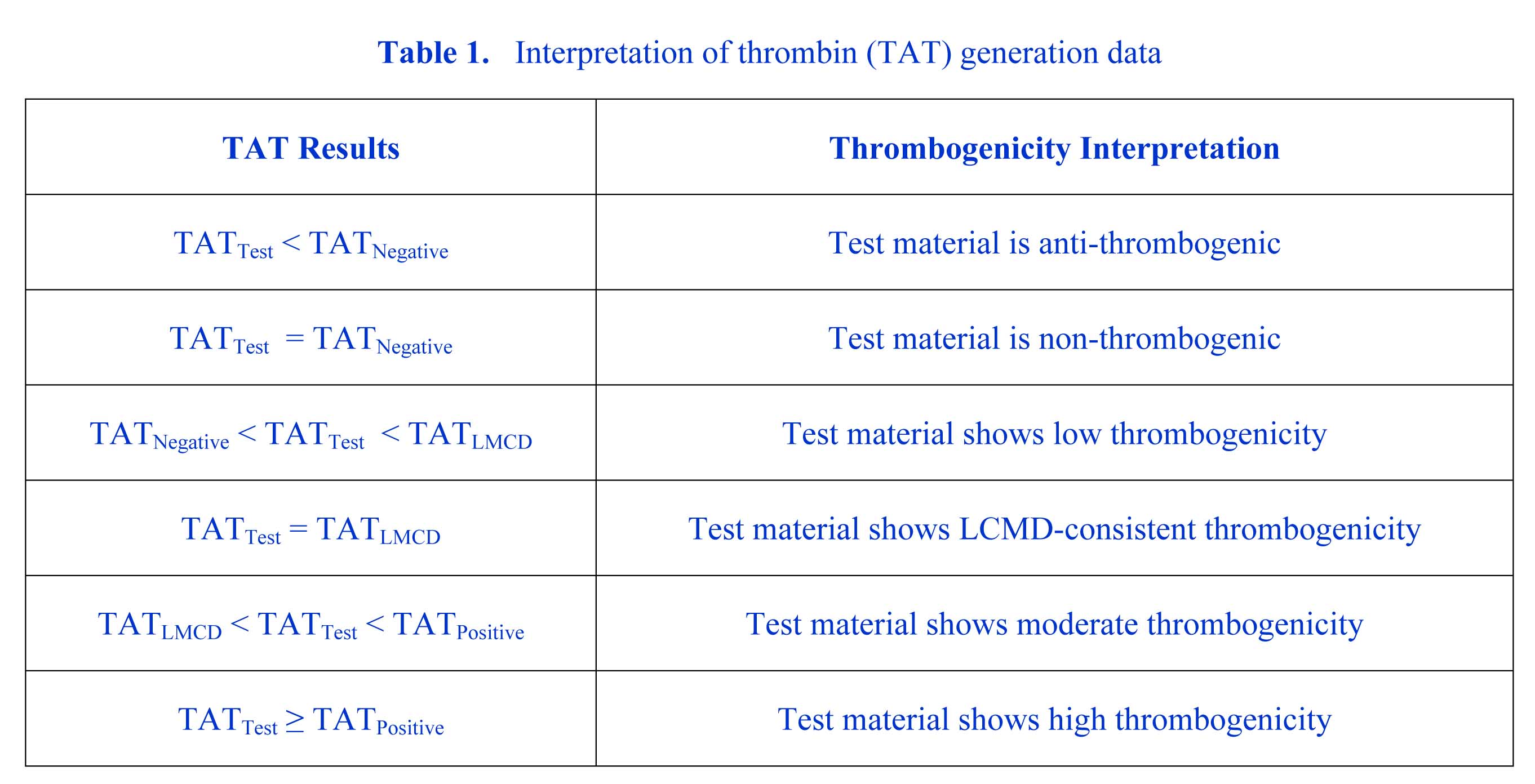
In the areas of basic research and early medical device development, the ISO10993-4 standard also serves as a useful reference guide for setting the approach, types of tests, and practical controls needed to define the clinically-meaningful hemocompatibility of a medical device material, material surface modification, or novel medical device design feature. For example, for an acute exposure e.g., = 1 hour blood contact device/material, the standard suggests that a combination of tests in the areas hemolysis and thrombosis, using a validated in vitro model simulating the clinical application, may be sufficient to characterize hemocompatibility safety. Here, thrombosis can be assessed by multifactorial approach using tests from a simplified list intended to capture the main elements of thrombosis e.g., thrombin activity, platelet reactivity, and immune system response. Variability and meaningfulness in measurements due to factors such as blood donor genetics and laboratory methods is addressed in the standard by the recommendation that all tests compare the Test material to a Negative control (e.g., system itself), Positive control (known high responder), and/or a legally marketed comparator device (LMCD) or LMCD material. Interpretation is then assessed objectively with standard statistical methods such as ANOVA and comparison of all means techniques, and simple evaluation criteria. Table 1 shows an example where the measured factor is thrombin generation assessed by TAT ELISA assay.
This presentation will give a high level overview of the 2017 ISO10993-4 standard. It will include examples of how the standard may be used in simple device cases. Other examples will be provided where the standard may be used in early development and characterization of novel materials intended to improve material/device hemocompatibility.
|
|
|
Contributed Talks
|
Characterization of Adsorbed Protein Orientation and Conformation
|
Robert A. Latour
McQueen-Quattlebaum Professor, Department of Bioengineering, Clemson University, Clemson, SC, USA
|
Protein adsorption on material surfaces is of fundamental importance for blood-biomaterial interactions. The structure and function of adsorbed proteins are tightly interrelated and play a key role in the bioactivity of the adsorbed proteins and their interactions with the surrounding environment. Because the bioactive state of a protein on a surface is a function of the orientation, conformation, and accessibility of its bioactive site(s), the isolated determination of just one or two of these factors will typically not be sufficient to understand the structure–function relationships of the adsorbed layer of protein. Rather a combination of methods is needed to address each of these factors in a synergistic manner to provide a complementary dataset to characterize and understand the bioactive state of adsorbed proteins. Over the past several years, my experimental group has focused on the development of a set of complementary methods to address this need. These methods include adsorbed-state circular dichroism spectropolarimetry to determine adsorption-induced changes in protein secondary structure and amino-acid labeling/mass spectrometry to assess adsorbed protein orientation and tertiary structure. In this presentation, I will provide an overview of the methods that we have developed and/or adapted for each of these assays. Examples of these methods will then be presented to demonstrate how they can be applied to investigate the molecular-level mechanisms that influence platelet adhesion response to various surface chemistries.
|
|
Studying Platelet Activation by Time-of-Flight Secondary Ion Mass Spectrometry with Principle Component Analysis.
|
Ilya Reviakine,1 Alessia Donati2
1 Department of Bioengineering, University of Washington – Seattle, Seattle, Washington, 98105, USA
2 Institute of Functional Interfaces (IFG), Karlsruhe Institute of Technology (KIT), Hermann-von Helmholtz-Platz 1,76344 Eggenstein-Leopoldshafen, Germany
|
Regulation of platelet activity holds the key to the delicate balance between haemostasis and thrombosis. On the one hand, these anuclear cell fragments circulating in blood catalyze clot formation at the site of injury—a mechanism for limiting traumatic blood loss. On the other hand, they are well-known for their sinister role in cardiovascular disorders (CVDs), the number one cause of death world-wide. They are also involved in a diverse variety of processes: wound healing, angiogenesis, maintenance of vascular wall integrity, pathogen recognition, and the development of cancer metastases. Understanding the mechanisms underlying the regulation of this diverse set of platelet functions remains challenging. Part of the challenge arises from the limited number of (immuno)labeling strategies that could be used to distinguish between differently activated platelets. Therefore, the focus of our group has become on developing new labels and label-free methods for studying platelet functional diversity. Here, we present the first results of ToF-SIMS/PCA analysis of fixed platelets, from different donors, activated with different agonist, or adsorbed on model biomaterial surfaces. Relatively small size of the platelets (~ 4 um in the resting state) represents a particular challenge. Application of different instrument operating modes to address this challenge will be discussed. We demonstrate that it is possible to distinguish between platelets activated in different ways, paving the way for further studies.
|
|
Mechanism of Thrombus Repellency on Slippery, Liquid-Immobilized Surface Coatings.
|
Sally Gao1,2, Anna Waterhouse1,2
1 Heart Research Institute, Newtown, NSW 2042, Australia
2 Charles Perkins Centre, Sydney Medical School, University of Sydney, Sydney, NSW 2006, Australia
|
INTRODUCTION: Thrombosis caused by medical devices can be costly and fatal for a number of reasons, including; 1, Thrombosis can cause failure of device function requiring device replacement which can be expensive, or cessation of blood flow, which can be fatal, and 2, embolism of the thrombus can cause pulmonary embolism or stroke. Recently, slippery, liquid immobilized surfaces have been utilized to prevent thrombosis and biofouling by preventing surface adhesion of blood and pathogens. Specifically, tethered-liquid perfluorocarbon (TLP) coatings reduce fibrin polymerization and platelet adhesion and activation in vitro under static and blood flow conditions. In vivo, an extracorporeal circuit consisting of TLP coated medically approved tubing and cannulae, remained patent for at least 8 hours at 15L/hr of blood flow in a swine arteriovenous shunt model without the use of any antithrombotic medication (Leslie et al., 2014). However, the mechanism by which proteins and cells are repelled by TLP remains poorly understood. Here we explore how fibrinogen and blood cells interact with TLP surfaces.
MATERIALS AND METHODS: TLP coated wells were generated using previously described methods (Leslie et al., 2014) consisting of oxygen plasma activation, silanization with trichloro(1H,1H,2H,2H-perfluorooctyl)silane and heating at 60°C for 16 hours. Following application of perfluorodecalin, fluorescence and confocal microscopy of the interface was carried out using fluorescent fibrinogen or whole human blood containing fluorescent fibrinogen and NucBlue ReadyProbes (Invitrogen), obtained with ethical approval and informed consent in accordance with the Declaration of Helsinki.
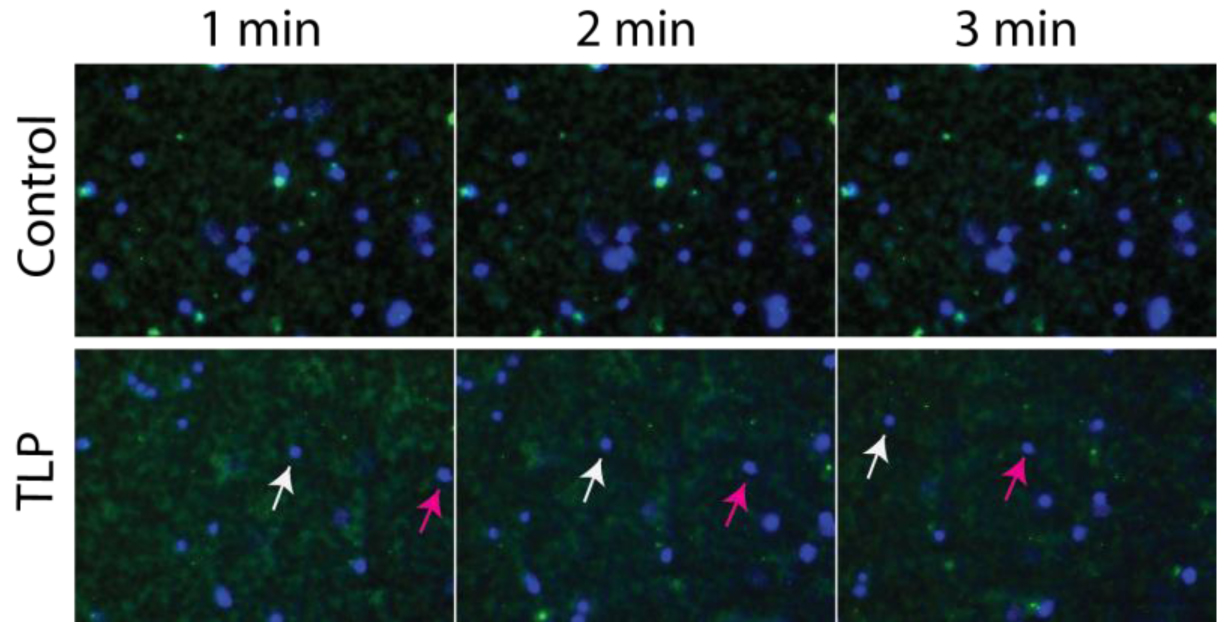
RESULTS AND DISCUSSION: Our results show that during a fluorescence microscopy time course, aggregates of a fluorescent fibrinogen solution adhere to wells over time and do not move compared to TLP-coated wells where the aggregates fail to settle in one location and move over time. Using live cell imaging, this same phenomenon occurs with whole blood, where blood components (nuclei and fibrinogen) settle and remain static on wells, compared to TLP coated wells, which continue to move over time without specific flow conditions (Figure 1). Confocal microscopy in the x, z planes revealed that this occurs even when the amount of liquid perfluorocarbon is below the limit of detection by microscopy (40X magnification). The TLP-blood interface is dynamic, showing reduced accumulation of blood components at the interface. This has implications for how thrombus propagation is reduced on TLP surfaces. Utilizing this system, the contribution of adhesion and local accumulation of blood components vs. protein and cellular activation to thrombosis and prevention of thrombosis could be elucidated.
CONCLUSIONS: TLP reduces the accumulation of blood components at the blood-liquid perfluorocarbon interface. Understanding the mechanism of the low-thrombogenic, repellent properties of TLP coatings will enable improved application to medical devices and provides insights for design improvements. Future studies will aim to determine the residence time and extent of activation of blood components at the perfluorocarbon interface.
ACKNOWLEDGEMENTS: This work has been supported by the Heart Research Institute, Australia.
REFERENCES: Leslie, D.C. et al. Nat Biotechnol, 2014, 32, 1134-1140
|
|
|
|
|
|
|
Figure 1. Fluorescence microscopy of whole heparinized blood on control (top panel) or TLP treated (lower panel) tissue culture plastic over a 3-minute time course (direction of movement on TLP: left to right). Magenta and white arrows indicate movement of 2 separate white blood cell nuclei.
|
|
|
|
Pursuing ultra-low VWF adsorption for blood-contacting devices under shear.
|
Wei Yang1,2, Junmei Chen1, Jennie Le1, José A. López1-5,*
1 Bloodworks Research Institute, Seattle, WA
2 Departments of Medicine; 3 Biochemistry; 4 Mechanical Engineering; and 5 Pathology, University of Washington, Seattle, WA
* Correspondence Email: josel@BloodWorksNW.org
|
Many human diseases are treated with blood-contacting medical devices.
However, they are often compromised because of blood proteins that accumulate on their surfaces. Such accumulation activates both platelets and blood coagulation, and patients supported by blood-contacting devices are at high risk of both bleeding and clotting.
Blood passing through medical devices is often subjected to high shear stresses (especially near surfaces) and flow acceleration, conditions that we have shown promote rapid deposition of the large plasma glycoprotein VWF onto uncoated surfaces.
VWF normally functions to initiate platelet attachment at sites of vessel injury.
Thus, its adsorption to artificial surfaces under shear is likely to initiate platelet binding and the adsorption of other plasma proteins, including clotting proteins.
Here we sought to create a surface that achieves ultra-low (<5.0 ng/cm2) VWF adsorption under shear which we anticipate will prevent further protein adsorption. To achieve this objective, we synthesized polymers from the zwitterion sulfobetaine methacrylate (PSB).
Various coating conditions for PSB were tested to achieve the lowest VWF adsorption.
After being exposed to a VWF solution under shear stress applied with a vortexer (3000 rpm, at RT for 90 min), VWF adsorption to polypropylene tubes coated under optimal conditions was below 2.6 ng/cm2.
The addition of methanol/dopamine is helpful to achieving a more uniform coating of the polymer on hydrophobic surfaces.
We further tested the stability of the PSB coating after long-term storage and extended shear time.
PSB-coated surfaces resisted VWF binding after 6 weeks in PBS at 4 ºC (<3.9 ng/cm2) and 6 hr of exposure under shear (<5.0 ng/cm2), indicating the long-term resilience of the PSB coating.
We then prepared a microfluidic device of polydimethylsiloxane (PDMS) that contained a tortuous flow channel and perfused purified VWF through devices that were either uncoated or coated with PSB.
FITC anti-VWF or citrated human whole blood containing platelets stained with calcein AM was then perfused through the devices to image VWF structure or platelet binding.
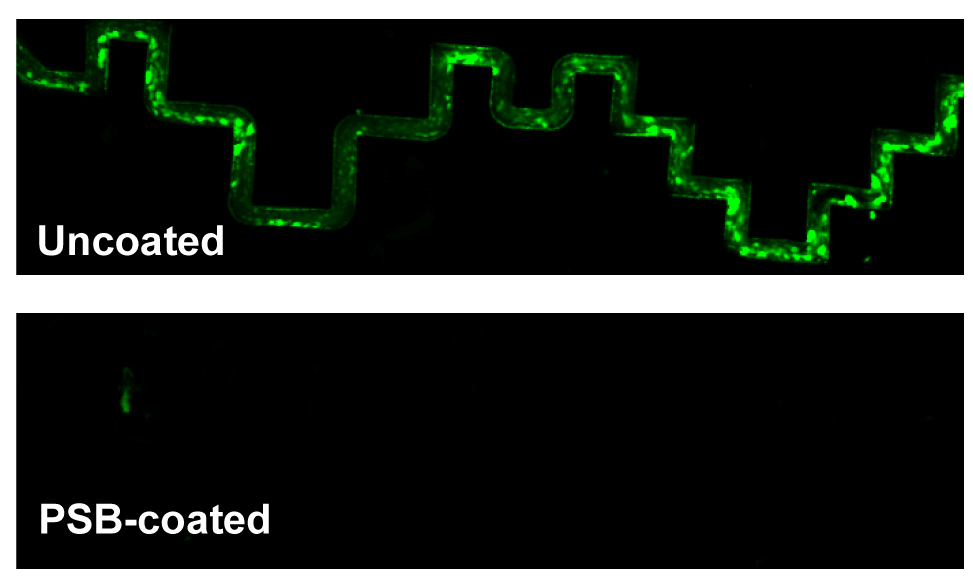
Under both conditions, VWF and platelet binding to the PSB-coated device was much lower than to the uncoated device.
On the uncoated device, VWF formed aggregates and bound platelets firmly when whole blood was perfused whereas in the PSB-coated device, VWF bound loosely and could be removed almost completely after wash.
In summary, polySBMA effectively prevented VWF adsorption under shear in two types of devices, and was stable during storage. We expect this coating, or a variation to significantly enhance the lifetime of blood-contacting devices and reduce both costs and medical complications.
|
|
|
|
|
|
|
|
Figure 1. Whole blood with calcein AM-labeled platelets was perfused through microfluidic channels with tortuous geometry, which were either uncoated or coated with PSB. At the end of perfusion, the channels were washed and fixed.
|
|
|








